Clinical Trial Equipment & Ancillary Solutions Market Future Scope and Latest Trends Analysis Report
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the “Global Clinical Trial Equipment & Ancillary Solutions Market Size, Share & Trends Analysis Report By Product (Sourcing, Supply/Logistics, Service, Others), By Phase (Phase I, II, III, IV), Region, Market Outlook And Industry Analysis 2031″
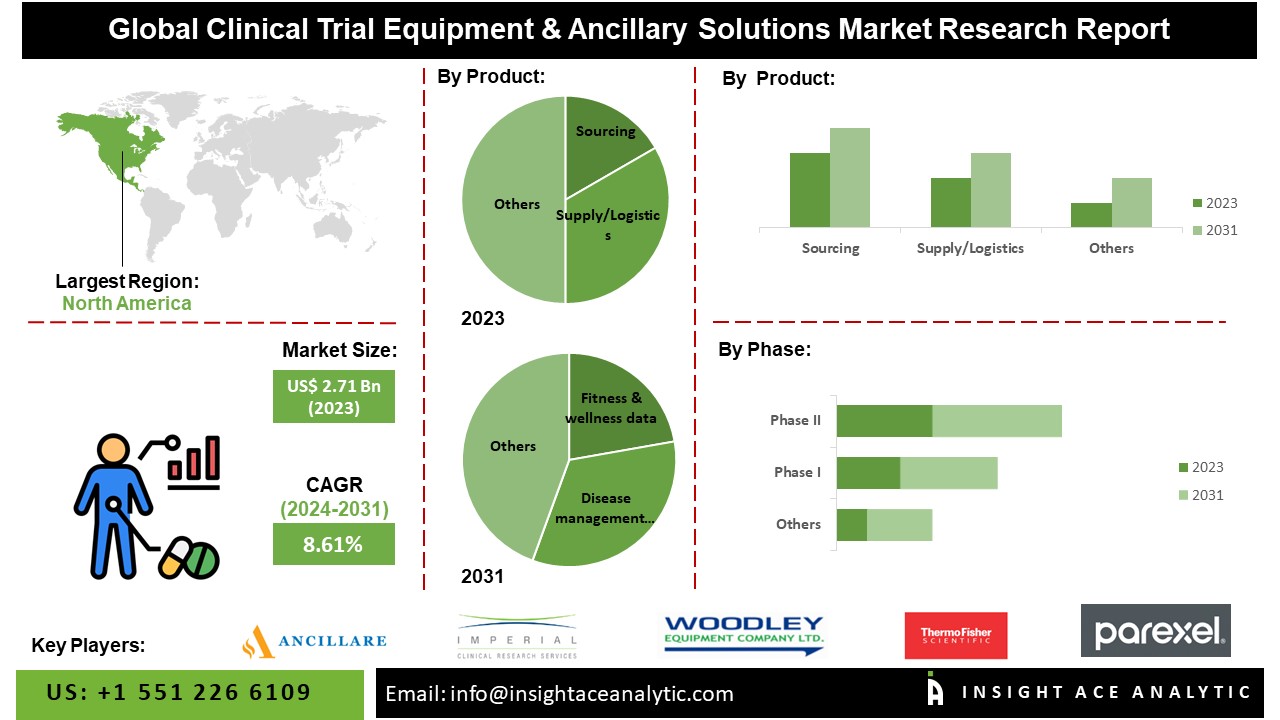
The global Clinical Trial Equipment & Ancillary Solutions Market is estimated to reach over USD 5.16 Bn by 2031, exhibiting a CAGR of 8.61% during the forecast period.
Request for Sample Pages: https://www.insightaceanalytic.com/request-sample/2326
The global market has experienced significant expansion propelled by increased investments in research and development, the growing necessity for clinical trials, and the rapid expansion of the pharmaceutical and medical sectors. In 2020, the progression of clinical trials was temporarily impeded by the COVID-19 outbreak due to government-mandated lockdowns, negatively impacting the market. Nevertheless, the heightened demand for effective treatments for COVID-19 and other ailments prompted a surge in trial numbers. In response to this growth, the company enlarged its facility by approximately 30,000 sq. ft., incorporating refrigerated and deep-frozen storage capabilities, along with expanded secondary packaging capacities. Consequently, significant players’ regional expansions are anticipated to enhance product accessibility. Companies are actively seeking out new technologies for investment, such as combinatorial synthesis, genomics, and proteomics, fostering innovation through the rigorous adoption of technological advancements in R&D.
Furthermore, businesses are allocating resources to research and development endeavors to discover novel chemicals and develop potential blockbuster drugs, resulting in a significant increase in generic drug manufacturers. There has been a notable uptick in R&D spending on orphan medications as they present opportunities for businesses to mitigate revenue losses stemming from the expiration of blockbuster drug patents. To address unmet needs and sustain profitability, companies are making substantial R&D investments, which are projected to drive demand for clinical trial tools and ancillary services.
Top of Form
List of Prominent Players in the Clinical Trial Equipment & Ancillary Solutions Market:
- Ancillary, LP
- Imperial CRS, Inc.
- Woodley Equipment Company Ltd.
- Thermos Fisher Scientific, Inc.
- Parexel International (MA) Corporation
- Emser (formerly Medi Capital Rent)
- Equipment SAS
- IRM
- Mirken
- Moyne
- Your way
Market Dynamics:
Drivers-
Increased investment in research and development projects is driving the market expansion for these goods. The devices and services mentioned earlier represent only a fraction of what the market for clinical trial devices and auxiliary services encompasses. As research and development efforts continue to increase, so does the demand for new tools and solution options. The future of this market looks promising, with the potential for groundbreaking solutions that will transform clinical trials. The global increase in clinical trials, propelled by advancements in medical research and drug development, is fueling demand for a wide range of equipment and ancillary solutions essential for conducting these trials. Growing emphasis on patient-centric clinical trials, prioritizing patient convenience, comfort, and participation. This emphasis has spurred the development of innovative equipment and solutions aimed at enhancing the overall patient experience during clinical trials.
Challenges:
With the rising expenses associated with clinical trials, the market’s growth rate for auxiliary services and equipment will be slower compared to the broader clinical trials industry. Designing equipment and solutions that prioritize patient comfort, convenience, and safety while meeting study objectives presents a unique set of challenges in the clinical trial equipment market. Logistical complexities in the supply chain, such as transportation and storage of sensitive materials, can pose significant challenges for companies operating in the market.
Regional Trends:
The North American Clinical Trial Equipment & Ancillary Solutions market is projected to mark a tremendous market share due to the abundance of key companies and sophisticated healthcare infrastructure in the region. The primary drivers of North America’s clinical trial supply market share are the heightened spending on Research and Development (R&D) by pharmaceutical and biopharmaceutical firms. Additionally, the growing presence of healthcare companies engaged in clinical trials in the region, supportive government regulations, and the accessibility of cost-effective products all contribute to this expansion. Besides, the Asia Pacific region has emerged as a prime location for clinical trials due to its advantageous regulatory compliance, cost-effectiveness in studies, burgeoning patient population, and prestigious clinical institutions serving as sites. Because of an expanded patient population, increased healthcare spending, and greater government support, the clinical trial supplies industry in regions like China, Singapore, Malaysia, and India is thriving. This growth is fueled by major pharmaceutical companies outsourcing their drug development services to these countries. The region’s rapid expansion is due to the growing prevalence of chronic diseases, lower clinical trial expenses compared to Western regions, and heightened government efforts to facilitate clinical trials. Additionally, end-users of clinical materials and supplies are expected to bolster their presence in these markets to comply with stringent import requirements.
Curious about this latest version of the report? @ https://www.insightaceanalytic.com/enquiry-before-buying/2326
Recent Developments:
- In July 2021, Parexel revealed an innovative partnership with the Cancer Hospital Chinese Academy of Medical Sciences (CHCAMS) aimed at crafting patient-centered protocol designs and methodologies for decentralized clinical trials within China. This initiative also encompasses quantitative research efforts to improve the clinical trial journey for oncology patients.
- In July 2021, Parexel launched a new clinical trial supplies depot in China to ensure prompt access to supplies and medications for clinical sites and patients.
Segmentation of Clinical Trial Equipment & Ancillary Solutions Market-
By Product-
- Sourcing
- Supply/ Logistics
- Service
By Phase-
- Phase I
- Phase I
- Phase III
- Phase IV
By Region-
North America-
- The US
- Canada
- Mexico
Europe-
- Germany
- The UK
- France
- Italy
- Spain
- Rest of Europe
Asia-Pacific-
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
Latin America-
- Brazil
- Argentina
- Rest of Latin America
Middle East & Africa-
- GCC Countries
- South Africa
- Rest of Middle East and Africa
For More Customization @ https://www.insightaceanalytic.com/customisation/2326
Media Contact
Company Name: InsightAce Analytic Pvt. Ltd
Contact Person: Diana D’Souza
Email: Send Email
Country: United States
Website: https://www.insightaceanalytic.com/